![PDF] Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience | Semantic Scholar PDF] Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8475108e210007edf6f24a80c253692db913891f/2-Figure1-1.png)
PDF] Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience | Semantic Scholar

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect
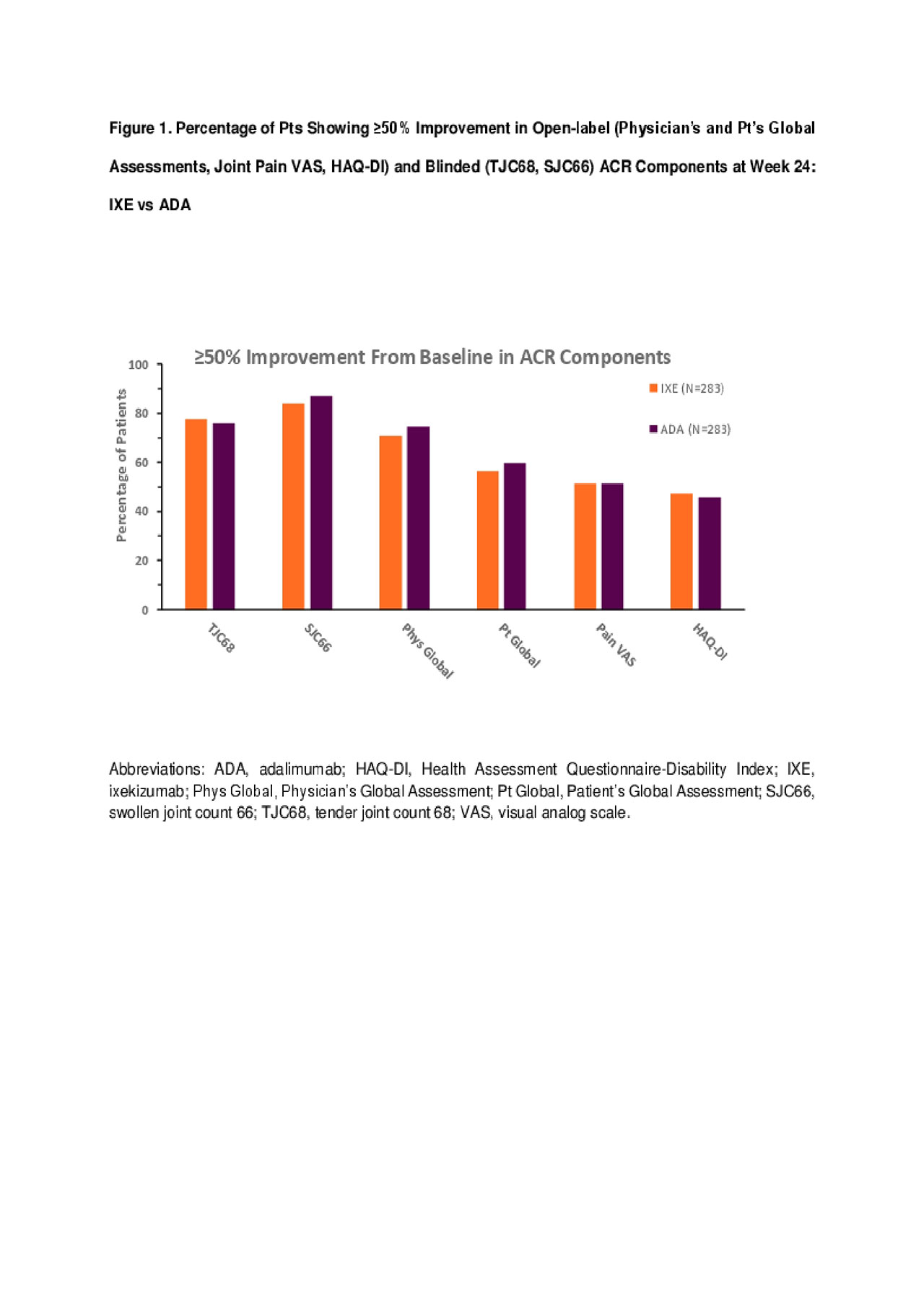
Ixekizumab Demonstrates Improvement Comparable to Adalimumab Across ACR Components in Biologic-Naïve Patients with Psoriatic Arthritis - ACR Meeting Abstracts

Lilly's Taltz® (ixekizumab) is the First IL-17A Antagonist to Receive U.S. FDA Approval for the Treatment of Non-Radiographic Axial Spondyloarthritis (nr-axSpA)
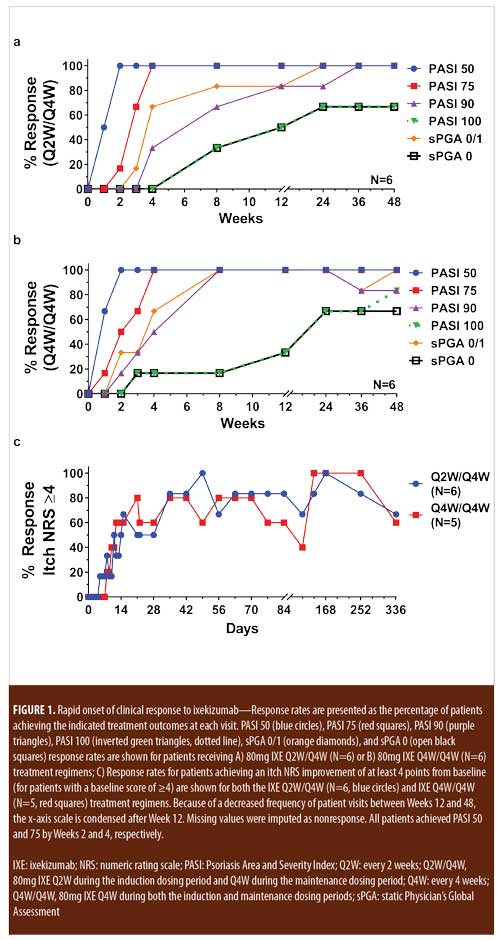
Early Onset of Clinical Improvement with Ixekizumab in a Randomized, Open- label Study of Patients with Moderate-to-severe Plaque Psoriasis – JCAD | The Journal of Clinical and Aesthetic Dermatology
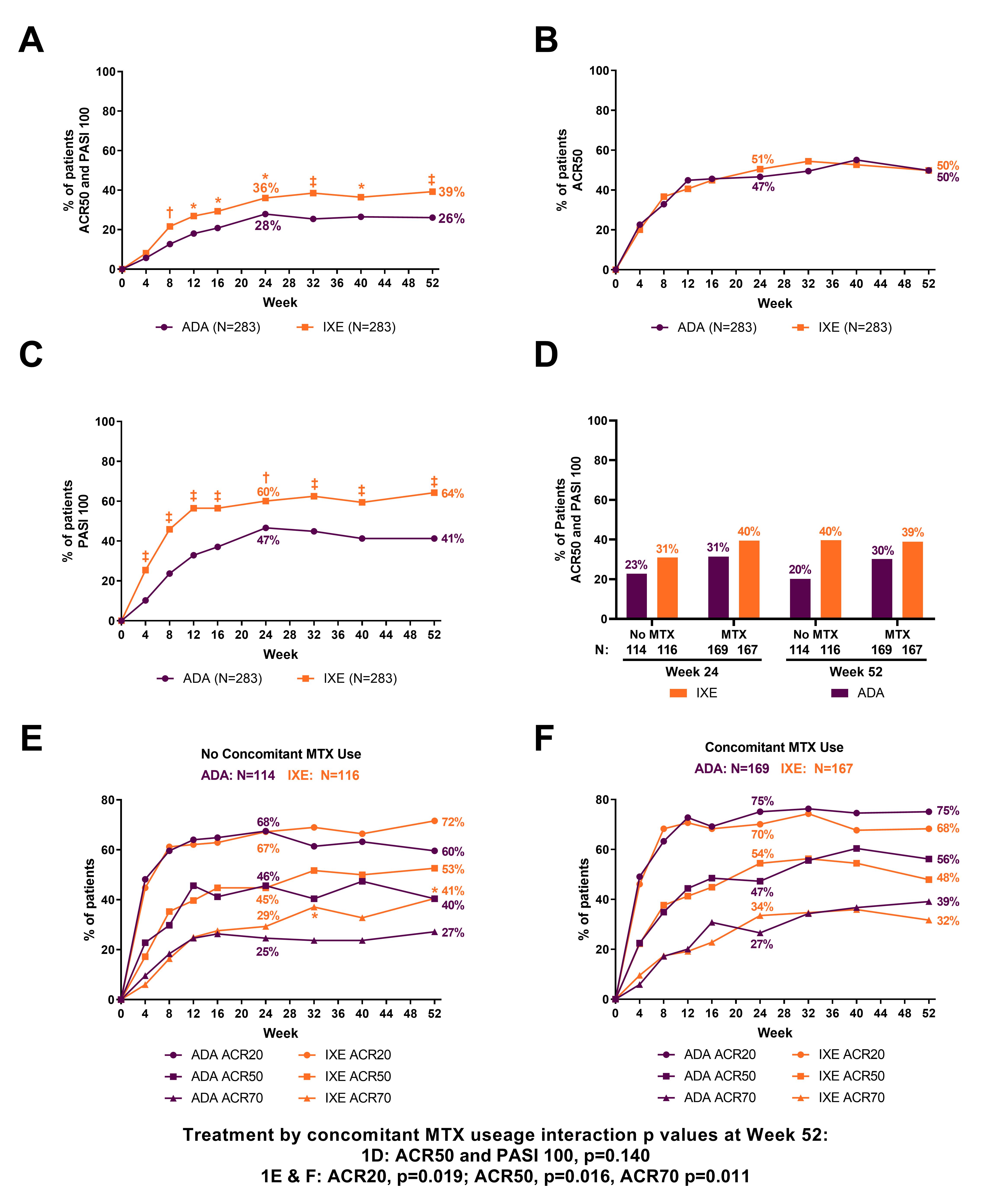
A Head-to-Head Comparison of Ixekizumab and Adalimumab in Biologic-Naïve Patients with Active Psoriatic Arthritis: Efficacy and Safety Outcomes from a Randomized, Open-Label, Blinded Assessor Study Through 52 Weeks - ACR Meeting Abstracts

A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis - ScienceDirect

LB0005 MULTICENTRE, RANDOMISED, OPEN-LABEL, ASSESSOR-BLINDED, PARALLEL-GROUP HEAD-TO-HEAD COMPARISON OF THE EFFICACY AND SAFETY OF IXEKIZUMAB VERSUS ADALIMUMAB IN PATIENTS WITH PSORIATIC ARTHRITIS NAIVE TO BIOLOGIC DISEASE-MODIFYING ANTI-RHEUMATIC ...

Figure 3. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology

PDF) Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: Results from the randomised, controlled and open-label phases of UNCOVER-3

Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial - The Lancet

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect

Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52 | Annals

Early Onset of Clinical Improvement with Ixekizumab in a Randomized, Open- label Study of Patients with Moderate-to-severe Plaque Psoriasis – JCAD | The Journal of Clinical and Aesthetic Dermatology

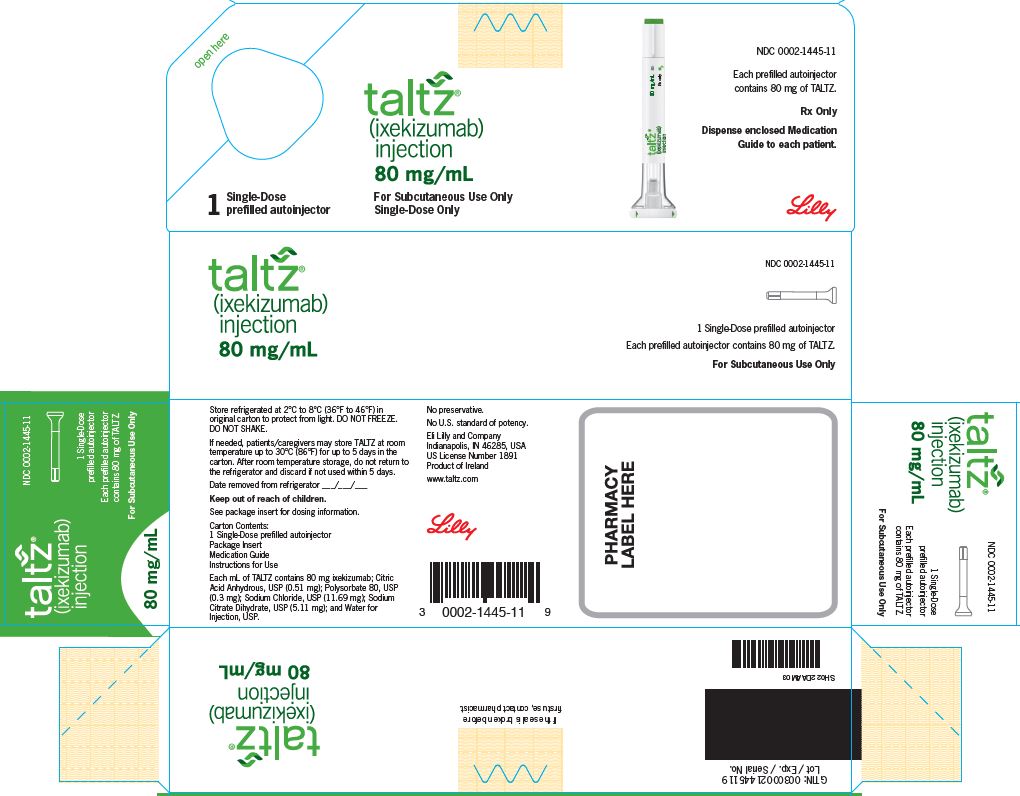
Fillable Online accessdata fda LABEL. TALTZ (ixekizumab) injection, Eli Lilly and Company - accessdata fda Fax Email Print - pdfFiller