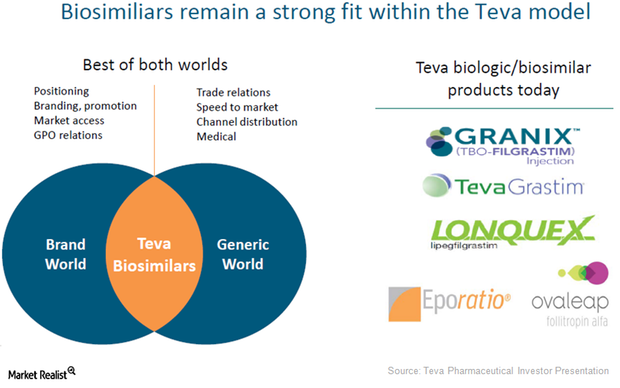
ACTAVIS: Teva Announces Updated Indication and Vial Presentation for GRANIX® (tbo-filgrastim) Injection in United States | American Pharmacy News

These highlights do not include all the information needed to use GRANIX safely and effectively. See full prescribing information for GRANIX.GRANIX® (tbo-filgrastim) injection, for subcutaneous use Initial U.S. Approval: 2012