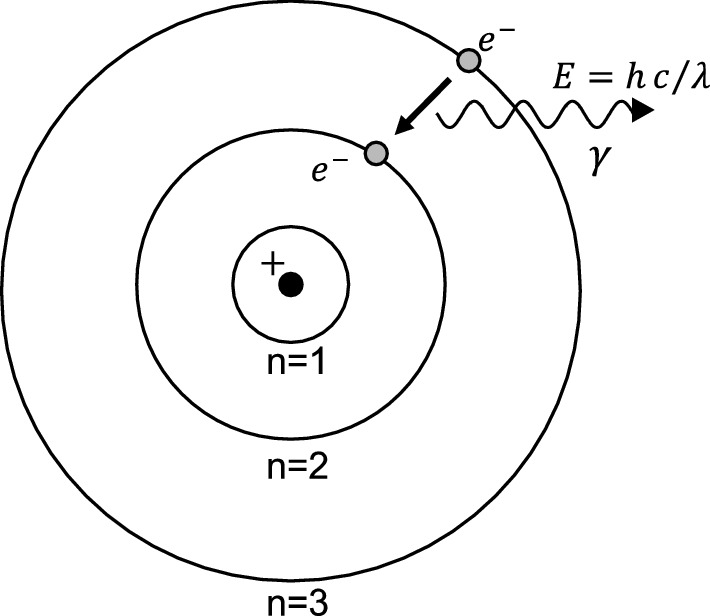
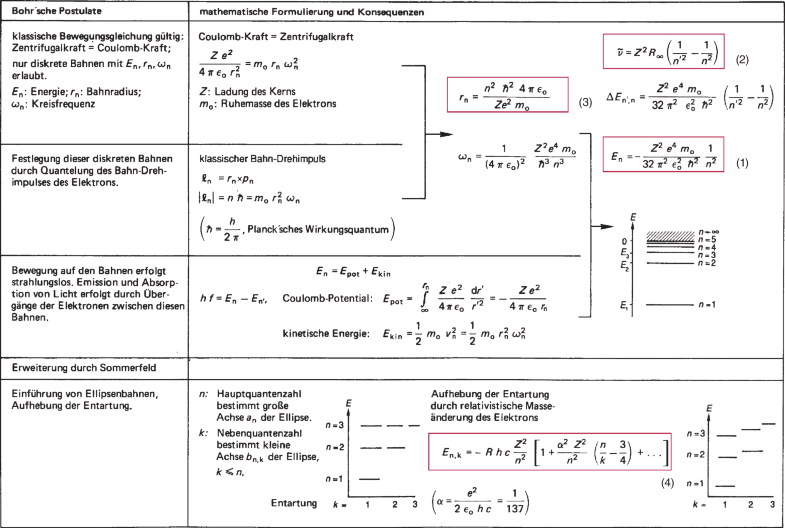
AS) 3. Give the main postulates of Bohr's model of an atom. (AS) Ctate the valencies of magnesium and sodium (AS)

PDF) Das Problem in ein Postulat verwandeln: Cassirer und Einsteins Unterscheidung von konstruktiven und Prinzipien-Theorien | Marco Giovanelli - Academia.edu

State Bohr's postulate of hydrogen atom which successfully explains the emission lines in the spectrum of

Boug 20 for 1 999 a fare 1. The electric potential energy of interaction between nucleus of an atom and an electron is given by U = 0, where r, is a